Promising Microbial Biopolymers: Sources, Genetics and Applications
Biopolymers
Biopolymers, which have become valuable for use in a lot of applications, are superior to derived of petrochemical in being biodegradable biocompatible and environmental.
The Polysaccharides are a different group of macromolecules found in nature and widespread. They can be disconnected due to their localization morphology as: part of the cytoplasmic membrane or located inside cell wall as intracellular polysaccharides or located outside cell wall as extracellular polysaccharides
Extracellular polysaccharides found in two structures: non-adherent to the cell which called loose slime and give it a sticky consistency to development of bacterial on a solid medium or in a liquid medium give a viscosity form and capsules or microcapsules, which hold to the cell wall. They have a distinct structure and boundary, being only gradually separated in the salt or water solutions. Therefore, it is possible to disconnect microcapsules and capsules by centrifugation from loose slime (Wilkinson, 1958).
Exopolysaccharides are long chain polysaccharides composed of repeating sugars branched units or derivatives of sugar, fundamentally galactose, rhamnose and glucose in different ratios. They are divided into two groups: heteropolysaccharides (xanthan and gellan) and homopolysaccharides (curdlan, mutan, cellulose, dextran and pullulan) (Sutherland, 1994). Homopolysaccharides composed of only one kind of monosaccharides (D-fructose or D-glucose) connected either by a combination of a set number of linkage types or by a single linkage type. While, Heteropolysaccharides composed of numerous oligosaccharides copies, include 3 to eight 8 residues, produced by a different microorganism. There are a lot of industrial applications for exopolysaccharides in pharmaceutical, food and other industries such as cosmetics, gelling agents, medicines for wound dressing, paper and textile (Sutherland, 1998).
The important examples in monosaccharides is cellulose. Cellulose is the most important biopolymers, is produced fundamentally by plants. However, a few bacteria species can also produce cellulose with a chemical formula like to that of plant cellulose but with unique physical properties. This variation is because of the reticulated network of their fine fibrils with diameter of (0.1m) is about 100th that of wood fibers. Also, unlike plant cellulose, BC does not need additional conversion to evacuated undesirable contaminants and impurities like as pectin, hemicellulose and lignin, in this way having the option to hold a prominent polymerization level (Nishi et al. 1990).
Bacterial cellulose
Bacterial cellulose (BC) has been recently a hallmark of several important biomedical products including vessel implants (tubes); wound care dressings; surgical threads; biocompatible implants; tissue reinforcement matrices besides other conventional industries like paper; food products; electronics and cosmetic products (Zhong, 2020).
Cellulose-producing bacterial strains
Also, microorganisms which produce cellulose have different applications and properties than that of plant cellulose. Cellulose is produced by different microorganisms like algae, bacteria and fungi. In green algae, mannan, xylan and cellulose serve as polysaccharides cell wall structural. Although, Cellulose is produced in small amount in most of the red algae (Rhodophyta), in all of the brown algae (Phaeophyta) and ultimate of the golden algae (Chrysophyta) (Richmond, 1991). In Oomycetes chitin is totally replaced by cellulose representing around 15 % of the wall dry mass. Also, Gram-negative species such as Alcaligenes, Azotobacter, Acetobacter, Sarcina, Rhizobium, Pseudomonas, Salmonella, Agrobacterium, Achromobacter, Aerobacter were produce cellulose (Morgan et al. 2013).
Synthesis of Bacterial cellulose
Bacterial cellulose synthesis is a exactly and specifically regulated many step process; as shown in Figure 5; containing a large number of both complexes of catalytic and regulatory proteins and individual enzymes, whose supramolecular structure has not yet been well known. Mechanisms and Pathways of uridine diphosphoglucose (UDPGlc) synthesis are comparatively well known, while mechanisms of glucose polymerization into long and unbranched chains still need searching (Prashant et al. 2009).
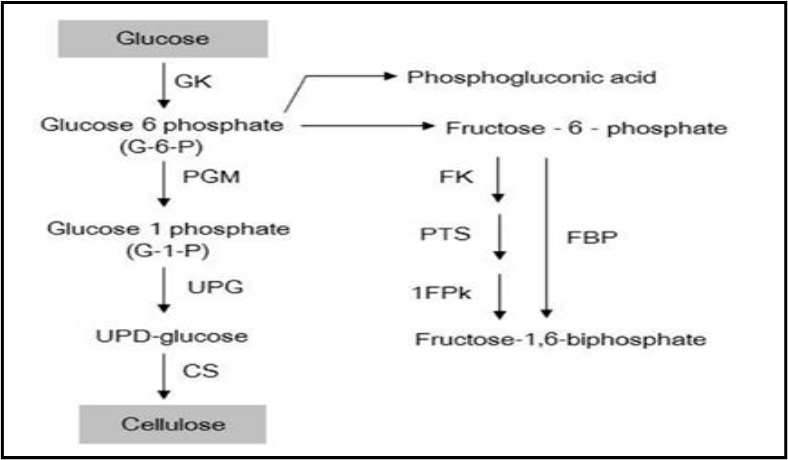
Figure: - The bacterial cellulose synthesis pathway of Acetobacter sp. 1FPk (fructose-1-phosphatekinase), PTS (system of phosphotransferase), FBP (fructose-1,6-bi-phosphate phosphatase), FK (fructokinase)
Bacterial cellulose structure
The molecular formula of bacterial cellulose (C6H10O5) n is the same as that of plant cellulose, but their physical and chemical features are different. Bacterial cellulose has a basic structure as a polymer strongly connected with a hydrogen chain between a hydroxyl group called microfibrils that has a thickness of 3-8 nanometres and width of 50-80 nanometres, which are composed of glucan chains which held together by intra- and inter- hydrogen bonding so that produced a crystalline domain. This structure microfibrillar of bacterial cellulose was first demonstrated by Mühlethaler, 1949 who proved that each bacterial strain produced different structures of cellulose.
Genes responsible for the production of microbial cellulose
In cellulose-producing bacterial strains; most prominent of which is Gluconoacetobacter hansenii, BC biosynthesis is orchestrated by four genes; namely A, B, C, and D comprising a 9.2 kb-long bacterial cellulose synthase (bcs) operon, preceded by two “regulatory” fragments reportedly essential for BC biosynthesis; cmcax gene that encodes an endo-β-1,4-glucanase enzyme that hydrolyzes BC and improves its synthesis, and ccpAx gene believed to play an important role in extracellular transportation of BC (Augimeri and Strap, 2015; Thakur et al., 2020). The main BC synthase (GDP-forming) enzyme (PF03552) is encoded by the bcsA fragment, while bcsB encodes a regulatory protein that belongs to the BcsB superfamily (pfam03170) encoding cellulose biosynthesis regulatory protein thought to bind the cyclic di-GMP positive effector (Yin et al., 2009); both comprise the complex glycosyl transferase unit responsible for transferring glucosyl residues from UDP-glucose to the 1,4-glucan chain (Knott et al., 2016). Meanwhile, the fragments bcsC (InterPro: IPR001440) and D (InterPro: IPR022798) are believed to be implicated in extracellular exporting and packaging of cellulose fibrils by forming a channel to the outer membrane allowing BC crystallization (Mehta et al., 2015). Several studies have reported that only bcsAB fragments are enough for effective in-vitro BC production (Omadjela et al., 2013); however, other studies confirmed the necessity of the four genes for maximum in-vivo production (Vijayendran et al., 2020).
The mass production of BC in different plasmid-based systems has been approached in several studies (Omadjela et al., 2013; Costa et al., 2017; Buldum et al., 2018). In this domain, E. coli platforms have been extensively used as superior protein producers for their rapid growth kinetics; ease of genetic manipulation, and rapid protein expression among other privileges (Sezonov et al., 2007). Among the most widely used bacterial hosts for recombinant proteins mass production are E. coli B strains [Rosetta and BL21 (DE3)] and K-12 strains including the main strain of plasmid replication DH5α (Marisch et al., 2013). However, as membrane-associated protein; cellulose synthase overexpression was reported to be toxic in E. coli BL21 (DE3), which paved the way for other alternative hosts like C41 (DE3); a mutant strain derived from BL21 (DE3) to study the possibility of mass expression of toxic membrane proteins of bcs-operon (Buldum et al., 2018).
Conclusion
Bacterial cellulose is an environmentally friendly, biodegradable biomaterial with unique properties that has qualified it to receive increasing attention for many industrial applications. However, mass production of bacterial cellulose using cellulose-producing wild type is still challenged by its slow growth kinetics, the large amount of glucose required for efficient production, and the high susceptibility to spontaneous mutations associated with fluctuations in nutrient/growth culture conditions that render it unproductive. Mutations, reduced cellulose productivity.
